Dialysate Filter Fit for Hemodialysis Device Produced
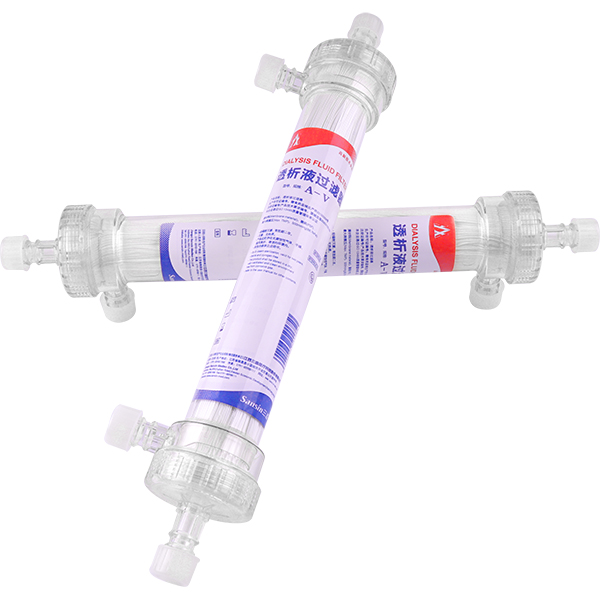
Hemodialysis Dialysate Filter Photo


Specifications
A-I,A-II,A-III and A-IV
Features
Sanxin Medtec produces high quality dialyzers for all your dialysis needs,fitting most of bloodline and dialysis equipments. Each manufacturing step is strictly controlled from plastic formulation to final sterilization.
Specially made membrane: The hollow fiber filtering membrane is customized for the dialysate filter, and it has excellent biocompatibility and strong endotoxin retention capability.
1. Significantly reducing and improving the patient’s micro-inflammatory reaction.
2. Reducing β2 microglobulin level and dialyze amyloidosis.
3. Increasing the sensitivity to EPO and protecting residual renal function.
4. According to customers’ requirements, we can produce what you need.
Professional: With Professional engineers & technicians & sale team.
Now are being leading exporter and distributor from China of medical devices for global customers.
Quality Assurance: Professional Inspector, control quality of each order. ISO9001:2008; ISO 13485:2003 certified manufacturing facility.
Ethical business dealings: Clients satification survey per month.
Company Belief: Sanxin to be your package solution.
Packaging
| Product | Size | Package Material | Volume | Carton Size | Measurement (ctns) |
Weight (kgs) |
MOQ (sets) |
||||
| Primary Package | Middle Package | Outer Package | PCS /carton |
20GP | 40HQ | N.W. | G.W. | ||||
| Dialysate Filter | A-I | PE |
/ | Carton | 100 | 66*38*42 | 250 | 610 | 5.5 | 9 | 20000 |



Certifications

Company Profile
Jiangxi Sanxin Medtec Co., Ltd., stock code: 300453, was founded in 1997. It is a national high-tech enterprise specializing in medical device R&D, manufacturing, sales and service. After more than 20 years of accumulation, the company has a global perspective, closely following national development strategies, closely following clinical needs, relying on a sound quality management system and mature R&D and manufacturing advantages, and has taken the lead in the industry to pass the CE and CMD quality Management system and product certification and US FDA (510K) marketing authorization.